WTF is the anion gap?
The anion gap, that unassuming number lurking on an EUC panel, is often described vaguely as the 'difference between the amount of cations and anions in the blood', which if one examines further, makes no sense as this should always be zero if the blood is to remain electro-neutral (and if it weren't one can imagine the catastrophic sequelae that would ensue).
The addition of one simple word, or punctuation mark, can drastically alter the meaning of a sentence. For instance, "I like cooking my family and my pets" and "I like cooking, my family, and my pets" have very different meanings.
In the context of the anion gap, the version of the latter phrase would be 'the difference between the amount of measured cations and anions in the blood', a seemingly simple distinction, but crucial to the meaning of what the 'gap' entails.
The measured cations in the blood comprise of Na+ and K+ (although some omit the K+ believing it to be a minor contribution), while the measured anions consist of HCO3- and Cl-.
Producing the formula:
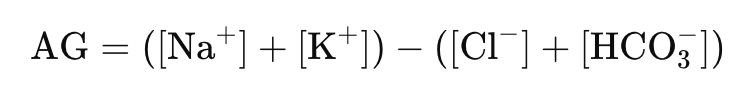
If there are measured cations and anions, it follows that there must also be unmeasured cations and ions in the blood.
In reality there are actually extremely few unmeasured cations in the blood, and their contribution to the total proportion is almost negligible.
However, there is a much more significant proportion of unmeasured anions in the blood (around 15% of total anions).
We know that:
Total cations = Total anions (to maintain electroneutrality)
Rearranging,
0 = Total cations – Total anions
0 = (Measured cations + Unmeasured cations) – (Measured anions + Unmeasured anions)
However the amount of unmeasured cations are virtually negligible. Thus:
0 = Measured cations – (Measured anions + Unmeasured anions)
Rearranging:
Unmeasured anions = Measured cations – Measured anions
However we said before that:
AG = Measured cations – Measured anions
Thus the anion gap is really just the amount of unmeasured anions in the blood, i.e. any anions that are not Cl- or HCO3-.
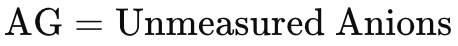
The AG is normally between 4 to 12 mmol/L.
The unmeasured anions include:
- Albumin and other plasma proteins
- Various organic anions including phosphate, sulfate, lactate, and ketones.
Any process that causes an increase in the number of unmeasured anions will increase the anion gap.
Anion gap and metabolic acidosis
The anion gap is most commonly used in the context of metabolic acidosis.
Metabolic acidosis can be categorised into high anion gap (HAGMA) and normal anion gap (NAGMA), which provides some clue into its likely origin.
In HAGMA there is accumulation of organic acids, such as lactate, ketones, or uraemic products (i.e. retention of sulfate and phosphate), as well as toxins such as methanol -> formic acid, which increase the proportion of unmeasured anions in the plasma. At the same time, these acids consume bicarbonate, lowering the amount of measured anions. Essentially, the body's buffer is being used up to to neutralise the excess acid.
In NAGMA, there is a loss of bicarbonate, either renally (i.e. Type 2 RTA) or from the gastrointestinal tract (i.e. diarrhoea/vomiting), but chloride rises to compensate to maintain electro-neutrality (which is why sometimes NAGMA is referred to as hyperchloraemic metabolic acidosis), keeping the overall anion gap normal.
Thus NAGMA responds to bicarbonate therapy because it directly replaces the lost base and corrects the acidosis.
In HAGMA although bicarbonate levels will be low, this is because it is being consumed as it buffers excess acid. However, the primary cause of the acidosis is not bicarbonate loss, so bicarbonate replacement is not typically used as a treatment of HAGMA (unless in severe cases). Replacement may temporarily buffer the acid, but it does not remove the excess acids, i.e. the root cause of the acidosis. Thus treatment should target the underlying cause, i.e. insulin for DKA, dialysis for uraemia.
While various acronyms to remember the causes of NAGMA and HAGMA exist, i.e. MUDPILES for HAGMA and CAGE of NAGMA (the internet is saturated with various iterations of these so it will not be repeated here; the interested reader can look these up), these are simply example of a much simpler principle.
This author believes it is more useful to understand the core difference between the causes of NAGMA and HAGMA, as this understanding will naturally allow one to think of causes without relying on rote memorisation (although acronyms are helpful as a secondary tool).
Limitations/Caveats
Other factors unrelated to acid/base disease can affect the anion gap, potentially masking elevation.
As mentioned earlier, albumin is a significant contributor to the unmeasured anions. Hypoalbuminaemia, which is common in critically ill patients, will therefore reduce the amount of unmeasured anions, and thus the anion gap.
Furthermore in some cases the proportion of unmeasured cations may become significant, such as in the case of multiple myeloma where there is an excess of positive charged paraprotein, or such as in lithium toxicity where there is an excess of lithium cations. This means that proportion of measured cations will reduce to remain electroneutrality, and this will reduce the overall anion gap which we remember is calculated by measured cations – measured anions.
Distilling these examples down to a principle, elevated unmeasured cations will reduce the anion gap. (Unmeasured cations cannot be decreased further because we are starting out with an already negligible proportion of unmeasured cations). Elevated unmeasured anions will increase the anion gap. Decreased unmeasured anions will decrease the anion gap.