Measurement of absolute lung volumes: Gas dilution and whole body plethysmography
Updated 12 Aug 2025 - 23:19
What are absolute lung volumes?
Absolute lung volumes are all the lung volumes that are NOT relative.
Basically Residual Volume (RV), which is the volume remaining in the lungs after a maximal forced expiration, and any lung capacity that incorporates RV.
These include:
- Total Lung Capacity (TLC), which is the total volume in the lungs after a maximal inspiration.
- Functional Residual Capacity (FRC), which is the volume in the lungs after a tidal volume expiration.
We can't measure absolute lung volumes using spirometry, as spirometry only measures changes in volume, i.e. the relative lung volumes.
Instead, we use one of the following two methods:
- Gas Dilution/Nitrogen Washout Test
- Whole body Plethysmography
Note: the gas dilution test only measures communicating gas. In gas trapping, the true lung volume will be underestimated. Whole body plethysmography is able to capture both communicating and non-communicating gas.
Gas Dilution/Nitrogen Washout Test
Allows us to work out FRC/RV based on how nitrogen in the lungs is diluted when a subject breathes in nitrogen free gas from a bag with a known volume.
The percentage of nitrogen in atmospheric air is approximately 79%. As nitrogen is an inert gas, the lungs are assumed to contain 79% nitrogen as well.
The subject starts out with FRC or RV in the their lungs, whatever volume we wish to measure. I.e. for FRC they would start at the end of a normal tidal volume expiration, whereas for RV they would start at the end of a forced maximal expiration.
This FRC/RV is assumed to have 79% nitrogen.
The subject then rebreathes a number of times from a bag of known volume, however many times one may reasonably assume it takes to allow the nitrogen present in the lungs to mix with the nitrogen-free gas in the bag, until the concentration of nitrogen in the lungs and the bag equilibrates.
We can measure the concentration of nitrogen in the closed system (comprising of the subject's lungs and bag) once it has equilibrates. We also already know the volume of the bag. Using this information, we can calculate the volume that was originally in the lungs.
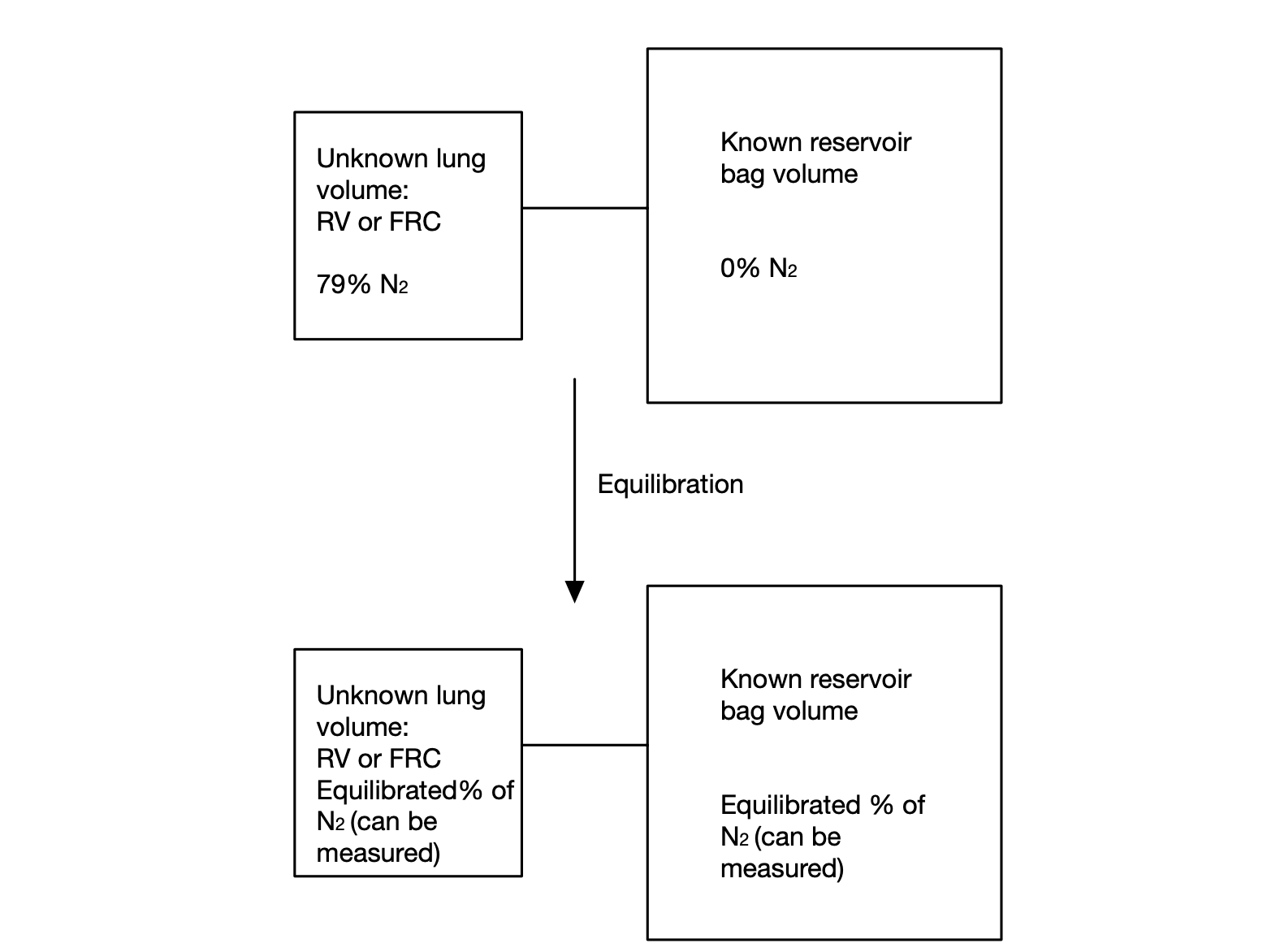
Obviously in a closed system the total amount of nitrogen will remain the same:
Total amount of nitrogen in the system initially = Total amount of nitrogen in the system after equilibration
Total amount of nitrogen in the system initially = 0.79 x lung volume
Total amount of nitrogen in the system after equilibration = % of nitrogen after equilibration x lung volume + % of nitrogen after equilibration x known reservoir bag volume
Thus
0.79 x lung volume = % of nitrogen after equilibration x lung volume + % of nitrogen after equilibration x reservoir bag volume
0.79 x lung volume - % of nitrogen after equilibration x lung volume = % of nitrogen after equilibration x reservoir bag volume
lung volume (0.79-% of nitrogen after equilibration) = % of nitrogen after equilibration x reservoir bag volume
lung volume = (% nitrogen after equilibration x reservoir bag volume)/(0.79 - % of nitrogen after equilibration)
or more formally:
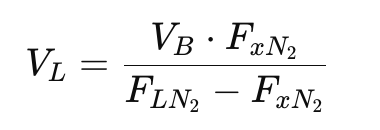
VL - lung volume (RV or FRC)
VB - reservoir bag volume
FxN2 - Fraction of nitrogen after equilibration (%)
FLN2 - Fraction of nitrogen initially in the lungs (which is 79%)
Because this method relies on air actually coming out of the lung and mixing with the gas in the bag, it follows that any nitrogen that is trapped behind obstructed airways, such as in COPD, will not be detected. The concentration of nitrogen measured in the system will be lower, underestimating the true lung volume.
Whole Body Plethysmography
Subject sits inside an sealed box of known volume. Pressure in the box can be measured.
The subject breathes through a breathing tube/mouthpiece that is initially connected to the outside air. The mouthpiece is equipped with a pressure detector, which detects pressure in the mouth, and when the system is closed (i.e. when the shutter closes), the pressure in the mouth will be equivalent to the pressure in the lungs.
The subject exhales to a particular volume again just as in the gas dilution test, either to FRC or RV, and then a shutter closes over the breathing tube.
The subject then attempts to breathe in against the closed shutter. This causes the intra-thoracic volume to expand, reducing the pressure of the air in their lungs.
The increase in their chest volume will then decrease the volume of what is in the box by the same amount. The pressure of the air in the box will increase as a result.
This is Boyle's law in action, which states that at a constant temperature in a closed system, the product of pressure and volume will be a constant, i.e. P x V = k where k is a constant.
So
P1lung (pressure in lungs initially) x V1lung (volume in lungs initially) = P2lung (pressure in lungs after inspiration against shutter) x V2lung (volume in lungs after inspiration against shutter)
rearranging gives
V1lung = (P2lung x V2lung)/P1lung.
We know P1lung and P2lung as they are measured at the mouthpiece. But we don't know V2lung.
How can we work out V2lung then?
Well we know that V2lung is just V1lung + ΔV (increase in volume in lungs)
so
V1lung = (P2lung x (V1lung + ΔV))/P1lung
V1lung x P1lung = P2lung x (V1lung + ΔV)
V1lung x P1lung = P2lung x V1lung + P2lung x ΔV
V1lung x P1lung - P2lung x V1lung = P2lung x ΔV
V1lung x (P1lung - P2lung) = P2lung x ΔV
V1lung = (P2lung x ΔV)/(P1lung - P2lung)
We can find ΔV, as it would also be the DECREASE in volume in the box (however much volume increases in the chest will displace volume in the box)
P1box (initial pressure in box) x V1box (initial volume in box) = P2box (pressure in box after inspiration against shutter) x V2box (volume in box after inspiration against shutter)
rearranging gives
V2box = (P1box x V1box)/P2box
but V2 box = V1 box - ΔV
so
V1box - ΔV = (P1box x V1box)/P2box
ΔV = V1box - (P1box x V1box)/P2box
Substituting this back into
V1lung = (P2lung x ΔV)/(P1lung - P2lung)
We obtain a rather complicated looking equation:
V1lung = (P2lung x (V1box - (P1box x V1box)/P2box))/(P1lung-P2lung)
or, LaTeX-ified:
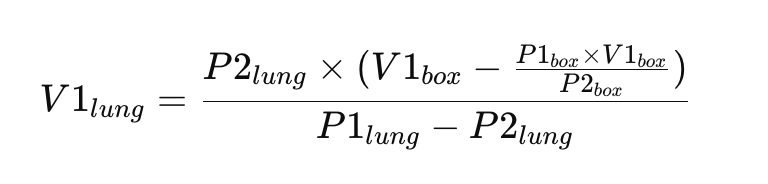
This will allow for the measurement of the total volume in the lung, regardless of gas trapping, so is more accurate than gas dilution in cases such as in COPD.
References
Wijayasiri L, Mccombe K. The Primary FRCA Structured Oral Exam Guide 1. 2nd ed. London: CRC Press; 2017.
Yartsev A. Measurement of lung volumes and capacities. Deranged Physiology: CICM Primary Exam – Respiratory System.